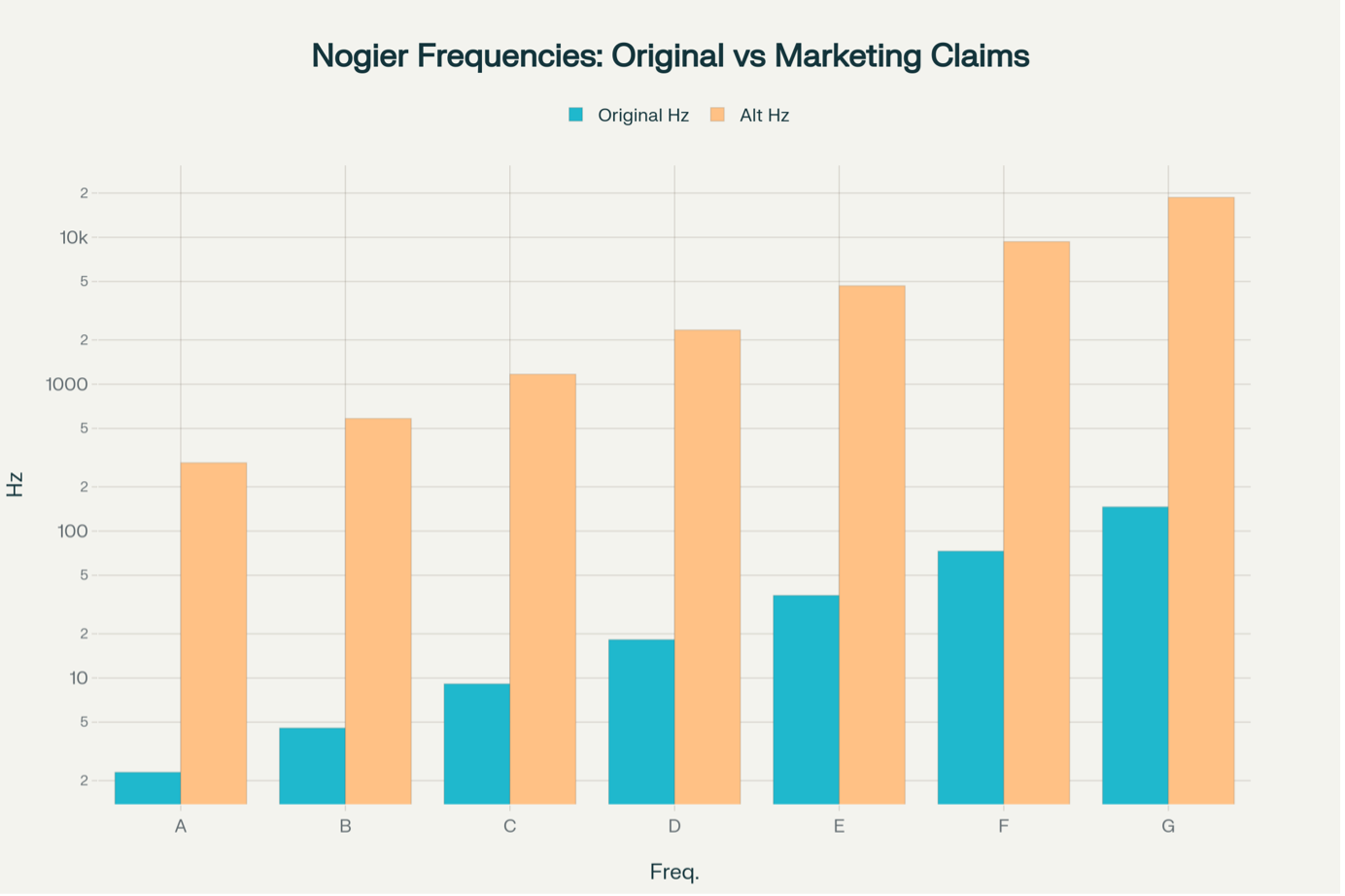
Nogier Frequencies and LED Photobiomodulation: Clearing Up the Confusion in a Rapidly Growing Industry
As Australia’s trusted leader in medical aesthetic technology, Aesthetic Bureau is committed to evidence-based solutions, transparency, and the highest regulatory standards. Here, we address the confusion surrounding Nogier frequencies in LED photobiomodulation. Our aim is to help you make informed decisions for your clinic and clients. Understanding the Nogier





